There is a massive explosion taking place in the space. The explosion is so immense that its radiation can destroy life from the earth in a matter of days. Earth will be doomed.
What if I told you this explosion took place 4.5 billion years ago and continue for the next 5 billion years. And there is a shield that has been protecting us from these ultraviolet rays just like captain America’s shield protects him from the enemies’ bullets.
The massive explosion is taking place at our beloved sun, where the nuclear fusion between the hydrogen nuclei takes place, forming the helium nucleus, causing massive dissipation of energy, which spread away in the form of electromagnetic rays.

These radiations from the sun are the source for almost all the energy on the surface of the earth. But it’s the radiations with a shorter wavelength (Ultra-Violet Rays) which are capable of destroying life from the earth’s face.
But to prevent the life on earth from getting extinct, there is an invisible protective shield in the atmosphere guarding us against these harmful rays. This shield absorbs these rays and dissipates them in the form of heat and thus maintains the earth’s temperature.
How do UV Rays affect us ?
For simplicity, we will divide the Ultra-violet rays coming from the sun into three categories Category A, Category B, and Category C, with A having the biggest wavelength and C the smallest.
UV-A rays
The Range of UV-A rays lies between 315 nanometers to 400 nanometers. The UVA rays have higher wavelengths but lowest in terms of energy compared to other wavelengths. These are penetrating cells and can affect the DNA of humans and animals. These rays can cause immediate tanning and often sunburns too. They can also cause premature aging, wrinkles, and long-term exposure can even cause cancer.
UV-B Rays
These Rays are harmful to both plants and humans. They have a shorter wavelength and can penetrate plants’ cells, easily damaging cell membranes and organelles within the cell, like mitochondria, chloroplast, etc. These damages to cells affect photosynthesis, respiration, growth, and reproduction. Which further leads to harming the crop yield and quality.
UVB is very biologically active but cannot penetrate beyond the superficial skin layers. Yet, experimental studies on animals and humans have shown that UV-B radiation may specifically suppress the functions of the immune system. It is responsible for delayed tanning and burning. It enhances skin aging and significantly promotes the development of skin cancer.
UV-C Rays
These are the most penetrable and have the most energy. Dead skin on most of the human body is sufficient to absorb UVC radiation almost completely. UVC radiation might reach live skin tissue and could produce erythema and possibly other undocumented effects.

So, what is protecting us ?
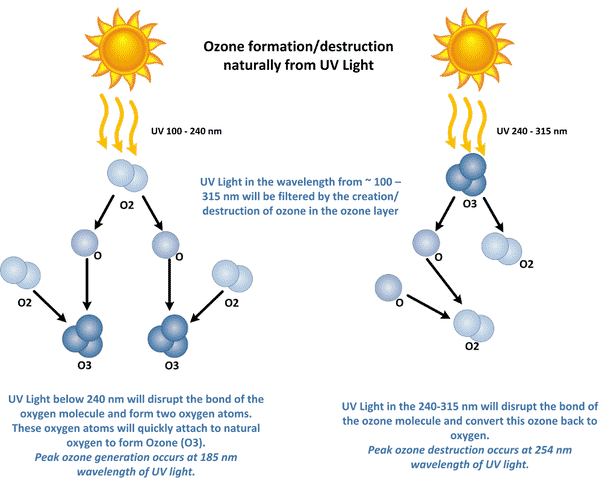
Ozone is an allotrope of oxygen made of three oxygen atoms; this allotrope of oxygen is present in abundance in our atmosphere at the height of about 15 to 30 Kilometers from the earth’s surface.

When the high energy UV light in the atmosphere reacts with the oxygen molecule, it disintegrates it into two oxygen atoms, further reacting with another oxygen molecule to form the three-atom allotrope of oxygen, called ozone. The ozone on reacting with UV light breaks into a normal oxygen molecule and the individual oxygen atom of oxygen. This is a continuing process known as the ozone-oxygen cycle.
O2 + ℎνuv → 2 O
O + O2 ↔️ O3
The UV-C rays cannot penetrate the Ozone layer, and Most of the UV-B rays are also blocked by the Ozone layer.
Thus, by this continuous Ozone-Oxygen cycle, the earth is protected from the more harmful UV rays and making the earth a habitable planet.
But this shield is about to break.
Due to several human activities leading to the release of pollutants in the atmosphere, the ozone layer is shrinking. Ozone depletion occurs when chlorofluorocarbons (CFCs) and halons—gases formerly found in aerosol spray cans and refrigerants—are released into the atmosphere. One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Ozone can be destroyed more quickly than it is naturally created.
Ozone depletion is most significant at the South Pole. It occurs mainly in late winter and early spring (August-November). Peak depletion usually occurs in early October, when ozone is often destroyed in large areas. This severe depletion creates the so-called “ozone hole.”
If this shield breaks, the large number of UV rays will start entering the atmosphere, the effects of which have already been discussed above. Due to the high amount of UV rays being able to reach the earth’s surface, they would react with the oxygen in the troposphere and start producing ozone allotropes, which can be fatal to living organisms.
What can I do to protect the shield?
The best way one can help protect the ozone layer is by reducing the use of ozone-depleting substances. You can take the following steps to make sure you are doing your part.
- Buy air-conditioning and refrigeration equipment that do not use CFCs as refrigerant.
- Buy aerosol products that do not use CFCs as propellants.
- Conduct regular inspection and maintenance of air-conditioning and refrigeration appliances to prevent and minimize refrigerant leakage.
- For existing air-conditioning and refrigeration appliances that operate on HCFCs or CFCs, the refrigerant should be recovered or recycled whenever an overhaul of equipment is to be carried out. Replacing or retrofitting such equipment to operate on non-CFCs refrigerant should also be considered.
- When motor vehicle air-conditioners need servicing, ensure that the refrigerants are properly recovered and recycled instead of being vented to the atmosphere.
Very informative post. Thanks for sharing 👍🏻
Thank you Sonali.
Keep update your blog !
I do wish to but have been busy lately.Hopefully i could continue soon.